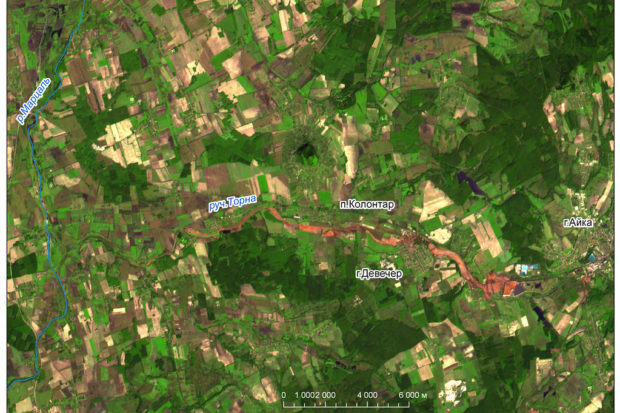
Shortly after noon on October 4, a toxic waste reservoir at the Ajka Timföldgyár alumina plant in western Hungary collapsed, releasing a “red river from hell”—a 3- to 7- foot-high tsunami of about a million tons (35 million cubic feet) of caustic sludge that initially flooded 15 square miles and several nearby localities, including the towns of Kolontár and Devecser.
Nine people died, and hundreds were burned by the highly alkaline mud (pH 13, hydroxide ion concentration a million times greater than that in neutral water) in the country’s worst ecological disaster. The spill reached the Danube, Europe’s second longest river, on October 7.
A spokesperson for MAL Hungarian Aluminium, which operates the plant, said that the last inspection of the reservoir before the accident had shown “nothing toward” and that it could not have predicted or been able to prevent the tragedy. MAL was so sure of the durability of the reservoir concrete that it did not have a protocol for responding to a breach. Sound familiar? Can you say BP?
The red mud gets its color from iron oxide (rust), which can constitute as much as half of the material. It also can contain arsenic, heavy metals and radioactive elements such as uranium or radium, depending on the mining site’s geographical location. A staggering 70 million tons of red mud is produced worldwide annually. Caustic sodium hydroxide (lye) which makes it so alkaline, is used to extract alumina (aluminum oxide) from the mineral bauxite. For every ton of alumina removed, the refining process produces about two tons of red mud.
Most refiners unload red mud into reservoirs open to the environment and let the material settle until the sodium hydroxide separates from the sludge and can be recovered for future refining or neutralized. When a reservoir fills, soil is often placed on top, greenery is planted and a new reservoir is built.
Other disposal methods have included dumping this by-product of the aluminum industry into the Mediterranean Sea or Pacific Ocean. Research on finding constructive, safe uses for red mud, from which toxic or radioactive components must be removed or stably contained, is still in its infancy. One proposed use is as an ingredient in bricks and roof tiles.
During the past four decades, at least 59 major failures of dams on refining slurry reservoirs have occurred globally. They have resulted in long-term damage to ecosystems, significant negative impact on nearby communities, and the loss of almost 700 lives.
The Hungarian toxic sludge disaster should serve as a caveat to us in the United States. From the deep South to the upper Midwest, there are thousands of open-air lagoons containing sewage from farms, feedlots and factory outfalls. A coal-ash dam that collapsed near Knoxville, Tennessee, in 2008 discharged more than a billion gallons of heavy-metal-laced ash—in volumetric terms, a bigger accident than the recent BP Deepwater Horizon spill in the Gulf of Mexico.
We can expect more such incidents. Securing frail retaining ponds has a low priority on a long list of tasks for the EPA and other environmental agencies, and global climate change resulting in more frequent floods intensifies the risk of catastrophic spills. The EPA and Congress should heed the timely lessons of Hungary and Tennessee to jump-start a process of sorely needed stronger protections and regulations. The preventive costs are only a fraction of the expenditures required to clean up after the disasters have occurred.
The Hungarian sludge catastrophe provides me with a unique opportunity to acquaint Community Alliance readers with some little known information about a common but much misunderstood metal.
Quick! What’s the commonest metal? If you answered “iron,” you’re wrong!
Aluminum is the third most abundant element and the most plentiful metal in the earth’s crust (7.5%). Combined with the two most abundant elements, oxygen (49.2%) and silicon (25.7%), it forms common clay. Together, these three elements constitute most (82.4%) of the rock on the earth’s surface. Although the production of aluminum is exceeded today only by that of iron and steel, metallic aluminum has been known for only little more than a century and a half. Not long ago, it was more expensive than silver and almost as expensive as gold.
Most people think that aluminum is not very reactive because it is used extensively in aircraft, cooking utensils, cans, wrapping, machinery, and countless household and industrial products. Exposed to air, a layer of aluminum oxide forms on its surface, which protects the underlying metal from further reaction, unlike iron, for which permeable oxide (rust) promotes further corrosion until the iron is entirely consumed.
CSUF student Matthew Adams and I published several lecture demonstrations vividly showing how active aluminum actually is. We treated aluminum foil with mercuric chloride. The metallic mercury that formed prevented the aluminum oxide from adhering to the aluminum so that it oxidized rapidly in the air.
Because of its extreme reactivity, aluminum is never found in the free state in nature, and its isolation from its compounds is extremely difficult and expensive. It was not until 1854 that a French schoolteacher, Henri Sainte-Claire Deville, prepared a small bar of aluminum, so precious that it was displayed next to the Crown Jewels at the Paris Exposition the following year. French Emperor Napoleon III hoped that aluminum could be used to make lightweight armor, used aluminum cutlery on special occasions, and had an aluminum rattle made for his young son.
Sainte-Claire Deville reduced the price of aluminum from $545 to $17 per pound, still far too high for widespread commercial use. However, he made the scientific world conscious of the need for a process to produce aluminum inexpensively and of the fortune that awaited whoever devised such a process.
We all know youngsters who, excited by raging hormones and plagued with inexperience, feign a braggadocio to mask their insecurity, appear to be supremely self-confident, and believe that they can do anything, but here’s a tale of two youths who actually did change our world.
The first commercially practical process for aluminum’s extraction was developed by 22-year-old Charles Martin Hall, who was inspired by his chemistry professor to search for a cheap production method. On February 23, 1886, eight months after his graduation from Ohio’s Oberlin College, Hall presented his former professor with the first buttons of aluminum prepared in an improvised woodshed laboratory by passing an electric current from homemade batteries through aluminum oxide dissolved in molten cryolite, an aluminum mineral. His process was a great commercial success, and between 1886 and 1914 the price of aluminum plummeted from $3 to 18¢ per pound.
In one of the great success stories of U.S. industrial research and development, young Hall became one of the founders of the Aluminum Company of America (Alcoa), which preserves these buttons, the so-called crown jewels, in its Pittsburgh headquarters. Alcoa is one of our nation’s 30 top public companies that comprise the Dow-Jones Industrial Index.
When Hall died, he left $5 million of his $30 million estate to his alma mater. Because of its light weight and resistance to corrosion, aluminum was used for the apex crowning the Washington Monument (1884) as well as for the plaque carried by Pioneer 10 (1972), the first manmade object to escape from our solar system. Today, aluminum has more than 3,000 common, everyday uses.
All of us are familiar with and perhaps uncritically accept the Great Man Theory of history popularized in the 1840s by Scottish satirist, essayist and historian Thomas Carlyle. According to this view, history can be explained largely by the impact of “great men” or heroes. However, a similar heroic theory that inventions and scientific developments are made by unique “geniuses” is less compelling. Multiple discoveries, made independently and simultaneously by different inventors and scientists, are fairly common. No wonder in science and technology we have so many priority disputes!
In a remarkable historical coincidence, on the 23rd of the same month (August) in the same year (1886), independently of Hall, young French metallurgist Paul-Louis-Toussaint Héroult (age 23) discovered essentially the same process for producing aluminum! He and Hall both were born in December 1863 and died in the same year, 1914. As Lord Byron cogently observed, “Truth is indeed strange; stranger than fiction.”
Perhaps today some high school or college science teacher is inspiring a young boy or girl to search for a cheaper method for producing another useful material that we use in our daily lives.
